Monsanto Catalyst
Monsanto Catalyst
The Monsanto catalyst is rhodium based catalyst for the manufacture of acetic acid by catalytic carbonylation of methanol. This process is known as Monsanto process. Homogeneous catalysis by transition metal complexes have become a major synthetic tool in industrial process. The potential of such catalytic reactions is due to the fact that most of these reactions are low energy processes. One of such metal complex system is rhodium (I)-complexes which play a big role as industrial homogeneous catalysts. The catalytic reactions span a wide range of complexes such as synthesis of fine chemicals like that of L-DOPA by an asymmetric hydrogenation of prochiral substrate with rhodium (I)-complexes continuing optically active ligands to synthesis of bulk chemicals like carbonylation of methanol for producing acetic acid by Monsanto’s process where as [Rh (CO)2 I2]–is the active catalytic species. Oxidative addition reaction is of great importance in inorganic and organometallic chemistry because of their application in hydroformylation of alkene. The hydroformylation of olefins is an extensively studied catalytic process and from an industrial point of view, one of the most important homogeneous catalysed reactions, since world-wide six million tons of aldehyde are produced each year. Starting 60 years ago heterogeneous catalyst were applied in the hydroformylation, followed by homogeneous cobalt and rhodium systems. In catalytic process the separation of products from the catalysts is still the main obstacle for commercial application.
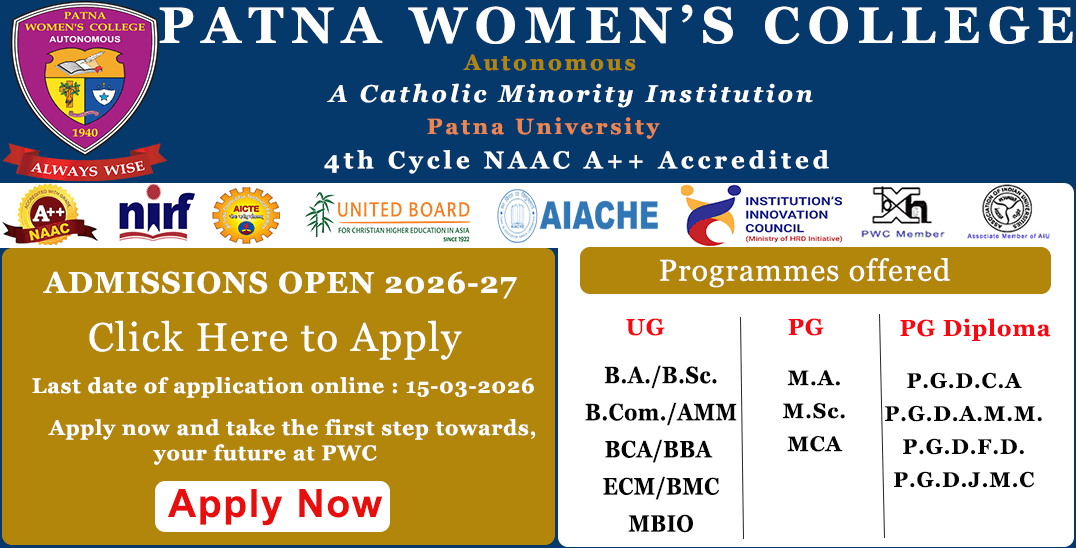
Methanol, which can be generated from synthesis gas (“syn gas”, a CO/H2 mixture), is reacted with carbon monoxide in the presence of a catalyst to afford acetic acid.
The carbonylation of methanol produces acetic acid:
Prior to 1970, acetic acid was made using cobalt catalysts (BASF process) requiring rather severe conditions. In 1970 Monsanto commercialized a rhodium carbonyl iodide catalyst that is commonly called the Monsanto Acetic Acid Process (developed in the late 60’s by James Roth and his research team at the corporate research centre in St. Louis). In 1986 Monsanto sold the acetic acid plant and technology to British Petroleum (BP), but it is still commonly referred to as the Monsanto Acetic Acid process.
Acetic acid is an important industrial commodity chemical, with a world demand of about 6 million tonnes per year and many industrial uses. The preferred industrial method for its manufacture is by the carbonylation of methanol and this accounts for approximately 60 per cent of the total world acetic acid manufacturing capacity. The carbonylation of methanol, catalysed by rhodium, was invented by Monsanto in the 1960s and for 25 years was the leading technology. In 1996 a new process for the carbonylation of methanol to acetic acid was announced by BP Chemicals, based on a promoted iridium catalyst package, named Cativa. The new process offers both significant improvements over the conventional rhodium-based Monsanto technology and significant savings on the capital required to build new plants or to expand existing methanol carbonylation units. Small-scale batch testing of the new Cativa process began in 1990, and in November 1995 the process was first used commercially, in Texas City, U.S.A.
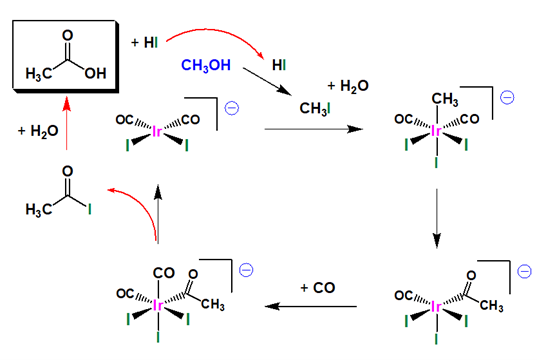
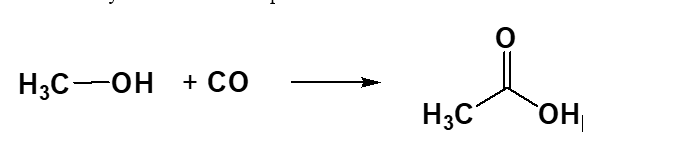
This process operates at a pressure of 30–60 atm and a temperature of 150–200 °C and gives a selectivity greater than 99%. The catalytically active species is the anion cis- [Rh (CO)2I2] −. The first organometallic step is the oxidative addition of methyl iodide to cis- [Rh (CO)2I2] − to form the hexacoordinate species [(CH3) Rh (CO)2I3] −. This anion rapidly transforms, via the migration of a methyl group to an adjacent carbonyl ligand, affording the pentacoordinate acetyl complex [(CH3CO) Rh (CO)I3] −. This five-coordinate complex then reacts with carbon monoxide to form the six-coordinate di carbonyl complex, which undergoes reductive elimination to release acetyl iodide (CH3C (O) I). The catalytic cycle involves two non-organometallic steps: conversion of methanol to methyl iodide and the hydrolysis of the acetyl iodide to acetic acid and hydrogen iodide. The reaction has been shown to be first-order with respect to methyl iodide and [Rh(CO)2I2]−. Hence the oxidative addition of methyl iodide is proposed as the rate-determining step.
References:
- J. Carberry, Chemical and catalytic reaction Engineering, Dover Publications, 2001.
Name: Dr. Nandini Kumari
Designation: Assistant Professor
Department: Chemistry