ORGANIC REACTIONS AND MECHANISMS
ORGANIC REACTIONS AND MECHANISMS
Organic reaction is a chemical reaction involving organic compounds. There are different types of organic reactions such as addition reaction, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions and redox reactions.
A reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. In today’s discussion, we shall restrict us only in substitution reactions.
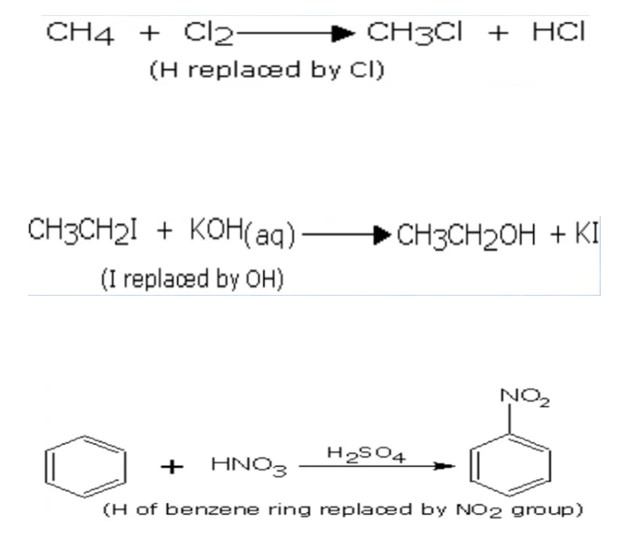
The reactions in which an atom or a group of atoms in a molecule is replaced or substituted by different atoms or group of atoms are called substitution reaction. E.g:
Substitution reactions are mainly of two types-
- Nucleophilic substitution (SN)
- Electrophilic substitution (SE)
We shall restrict today’s discussion in nucleophilic substitution only.
Nucleophilic substitution is a fundamental class of substitution reaction in which an “electron rich” nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom attached to a group or an atom called the leaving group; the positive or partially positive atom is referred to as an electrophile.
Nucleophilic substitution reactions can be broadly classified as;
- SN at saturated Carbon centers
- SN at unsaturated Carbon centers
Nucleophilic substitution at Carbon center: Edward D Hughes and Sir Cristopher Ingold studied nucleophilic substitution reactions of alkyl halides and related compounds. They proposed that there are two mechanisms, both of them competing with each other. The two main mechanisms are SN1 reactions and SN2 reactions. S stands for chemical substitution, N stands for nucleophilic and the number represents the kinetic order of the reaction.
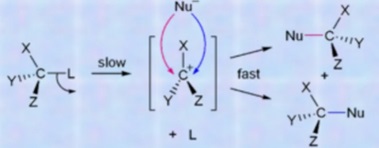
In the SN1 mechanism, the order of the reaction is 1. It means that in the slowest step of the reaction only 1 reacting species is involved. Hence, it is also called monomolecular reactions but the reaction takes place in 2 steps. The first step is slow step and the second step is the fast step. Thus we can say that SN1 reaction is a two-step reaction.
In SN2 reaction, the addition of the electrophile and the elimination of the leaving group takes place simultaneously. SN2 reaction occurs, where the central carbon atom is easily accessible to the nucleophile.
Thus SN2 reaction is a single step substitution reaction. By contrast, the SN1 reaction involves two steps.
SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups because such groups interfere sterically with the SN2 reactions because a highly substituted carbon forms a highly stable carbon cation.
Applications:
- Nucleophilic substitution via the SN1 or SN2 mechanism does not generally occur with vinyl or aryl halides or related compounds.
When the substitution occurs at the carbonyl group, the acyl group may undergo nucleophilic acyl substitution. Thus is the normal mode of substitution with carboxylic acid derivatives such as acyl chlorides, esters and amides.
Teacher’s Name: Rabindra Nath Roymajumdar
Designation: Assistant Professor
Department: Chemistry
E-Mail ID: royrabi11@gmail.com